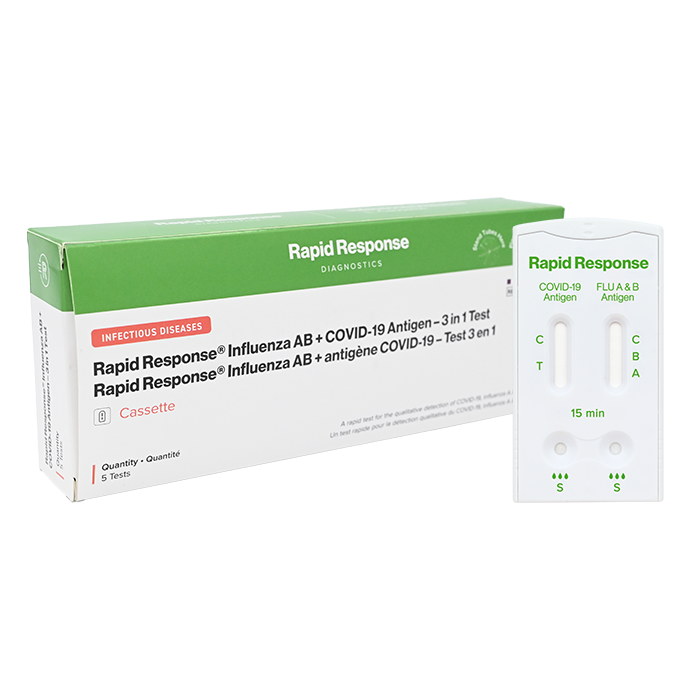
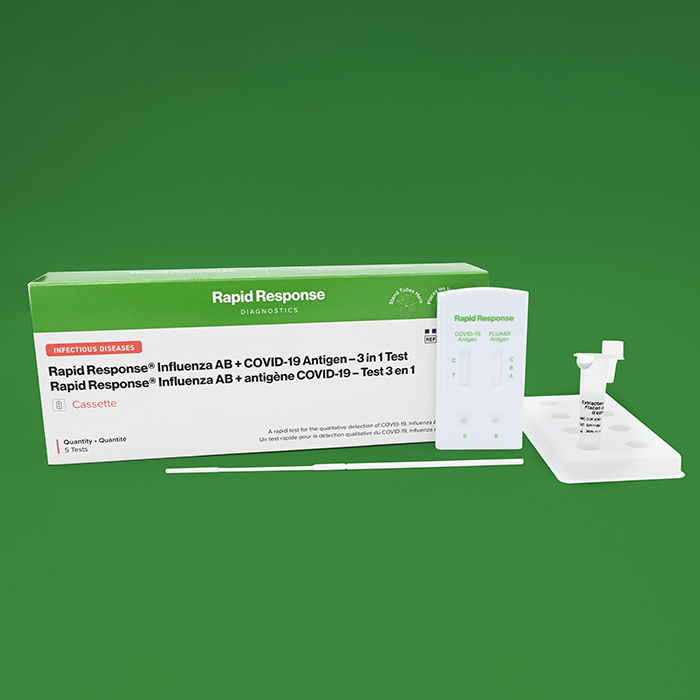
Influenza AB + COVID-19 Antigen – 3 in 1 Test
Influenza AB + COVID-19 Antigen – 3 in 1 Test
Product Information
Product Code: COF-19CPC5Sample: Nasopharyngeal or Anterior Nasal Swab Specimens
Format: Cassette
Quantity: 5 Tests
Sensitivity: Please contact us for details
Specificity: Influenza A/B and SARS-CoV-2
Accuracy: >98%
Time to result: 15 Minutes
Storage Condition: 2-30°C/36-86°F
Test Principle: Lateral Flow Immunoassay
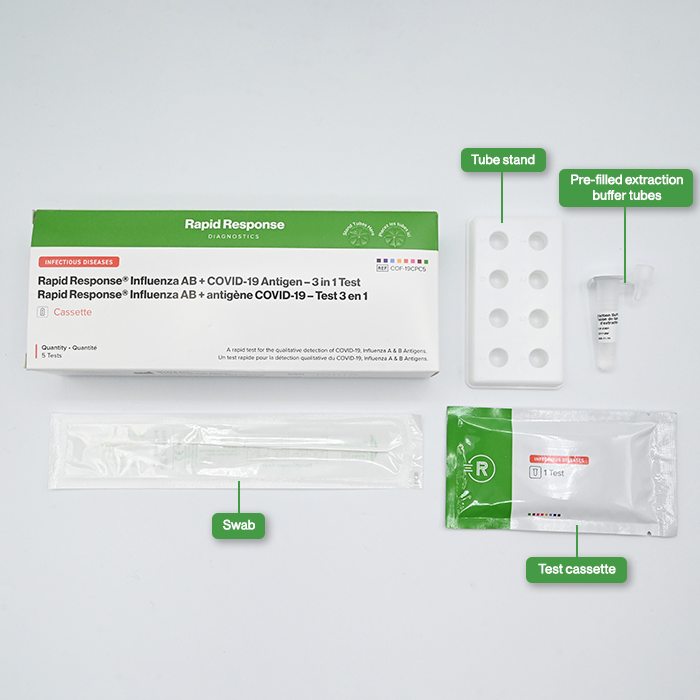
Contents
- 5 Individually packaged test cassettes
- 5 Pre-filled extraction buffer tubes
- 5 Disposable swabs
- 1 Product insert
- 1 Tube stand
Product Description
The Rapid Response® Influenza AB + COVID-19 Antigen Test is a rapid, lateral flow immunoassay for the detection and differentiation of nucleoprotein antigens from SARS-CoV-2, Influenza A and Influenza B in individuals suspected of COVID-19 or Influenza A or B within the first 7 days of symptoms onset. The test is intended for use by healthcare professionals and laboratory personnel trained to perform the test.
The Rapid Response® Influenza AB + COVID-19 Antigen – 3 in 1 Test is authorized under the Health Canada Interim Order (IO #356389). This test is suitable for use by healthcare professionals and laboratory personnel trained to perform the test.
- 3-in-1 Test: Detects antigens from three common viral respiratory infections: COVID-19, Influenza A and Influenza B - saving your team critical time during the busy cold and flu season
- Optimized Care: In just 15 minutes test patients from a single swab sample allowing your team to quickly determine the appropriate course for treatment during the same patient visit.
- Easy & Convenient: Uses non-invasive (anterior) nasal swab samples and the kit includes all materials needed to run the test.
- Streamlined Workflow: Integrates seamlessly into existing COVID-19 testing workflows with similar sample collection and processing procedure*
*See product insert (English, French)
FAQ
| A COVID-19 and Influenza Combo tests, such as the Rapid Response® Influenza AB + COVID-19 Antigen – 3 in 1, are singular Rapid Antigen Tests designed to identify the presence of three distinct antigens associated with COVID-19, Influenza A, and Influenza B infection. These antigens are generally detectable in anterior nasal or nasopharyngeal swab samples during the acute phase of infection. Each antigen has its own Test Line to indicate whether the sample being examined is positive or negative for the specific antigen it corresponds to, facilitating a clear differentiation between each ailment. One of the key advantages of a combo tests is its streamlined sample collection process. Combo test require just one sample, minimizing any potential discomfort for the patient associated with multiple swabbings. This streamlined approach not only enhances patient convenience but also provides a more comprehensive diagnostic profile compared to the standard single antigen tests. | |||||||||||||||||||||||||||
| The Rapid Response® Influenza AB + COVID-19 Antigen – 3 in 1 simultaneously detects and differentiating for SARS-CoV-2 (COVID-19), Influenza A, and Influenza B nucleoprotein antigens in nasopharyngeal/anterior nasal swab specimens. | |||||||||||||||||||||||||||
| Influenza (commonly known as ‘flu’) is a highly contagious, acute viral infection of the respiratory tract. It is a communicable disease easily transmitted through the coughing and sneezing of aerosolized droplets containing live virus.[1] Influenza outbreaks occur each year during the fall and winter months. Type A viruses are typically more prevalent than type B viruses and are associated with most serious influenza epidemics, while type B infections are usually milder. [1] Williams, KM, Jackson MA, Hamilton M. (2002) Rapid Diagnostic Testing for URIs in Children; Impact on Physician Decision Making and Cost. Infec. Med. 19(3): 109-111. | |||||||||||||||||||||||||||
| COVID-19 is an acute respiratory infectious disease caused by the SARS-CoV-2 virus, a novel Betacoronavirus. SARS-CoV-2 is mostly spread person-to-person, both by individuals with symptoms of COVID-19 infection and by infected people without symptoms. Based on the current knowledge, the incubation period is 1 to 14 days, mostly 4-5 days. Symptoms include fever, fatigue, and cough. For a full list of symptoms, see: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html | |||||||||||||||||||||||||||
| The Rapid Response® Influenza AB + COVID-19 Antigen – 3 in 1 Test is suitable for use by healthcare professionals and laboratory personnel trained to perform the test. The test is intended for individuals age 2 years of age or older who are suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider. Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2 and Influenza can be similar. | |||||||||||||||||||||||||||
| Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2 and Influenza can be similar. Symptoms related to COVID-19 or Influenza A/B within seven (7) days of the onset of symptoms, include fever, cough, unexplained loss of taste and smell, sore throat, headache, shortness of breath, muscle/joint ache, or diarrhea. | |||||||||||||||||||||||||||
| | |||||||||||||||||||||||||||
| The Rapid Response® Influenza AB + COVID-19 Antigen – 3 in 1 Test can be read 15 minutes after the sample is added to the buffer well. | |||||||||||||||||||||||||||
| A total of 421 individuals among multiple sites were enrolled to verify the performance of Rapid Response® Influenza AB + COVID-19 Antigen – 3 in 1 Test. To be enrolled in the study, subjects were either asymptomatic or presenting symptoms related to COVID-19 or Influenza A/B within seven (7) days of the onset of symptoms, such as fever, cough, unexplained loss of taste and smell, sore throat, headache, shortness of breath, muscle/joint ache, or diarrhea. The accuracy for each of the antigen tests is summarized below:
| |||||||||||||||||||||||||||
For additional information regarding any of our products, please feel free to Contact Us