
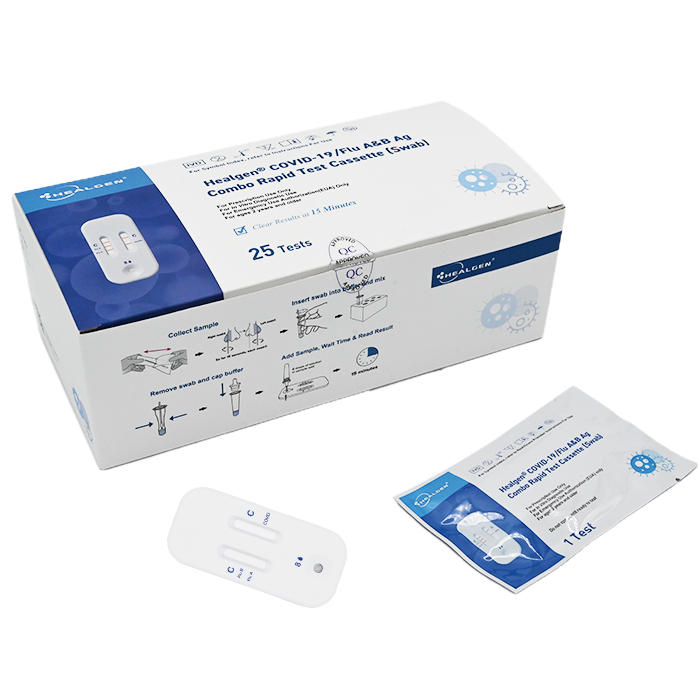













The Healgen® COVID-19/Flu A&B Ag Combo Rapid Test Cassette (swab) is a lateral flow immunochromatographic assay intended for in vitro rapid, simultaneous qualitative detection and differentiation of influenza A and influenza B nucleoprotein antigens and SARS-CoV-2 nucleocapsid antigen directly from anterior nasal swab specimens.
The Healgen® COVID-19/Flu A&B Ag Combo Rapid Test Cassette (swab) is intended for use in individuals who are suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider within the first five (5) days of symptom onset when tested at least twice over three days with at least 48 hours between tests.
Suitable for testing individuals 2+ years old. Swabbing should be performed by an adult for children aged 2 to 13. User must be aged 14 + to perform self test.
Authorized Laboratories: Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests. This product is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
| Product | Item Code | Quantity |
| COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab) | GCFC-525Sa | 25 Tests |
| External control: COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab) | GCFC-PN2 | 1 Each negative and positive control swab |
| External control: COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab) | GCFC-PN10 | 10 Each negative and positive control swab |
Features
- 3-in-1 Test: Detects antigens from three common viral respiratory infections: COVID-19, Influenza A and Influenza B.
- Optimized Care: In just 15 minutes, test patients from a single swab sample and quickly determine the appropriate course for treatment during the same visit.
- Highly Sensitive:
92.0% sensitivity, 99.0% specificity for COVID-19.
92.5% sensitivity, 99.9%specificity for Flu A.
90.5% sensitivity, 99.9% specificity for Flu B. - Easy & Convenient: Uses non-invasive anterior nasal swab samples and the kit includes all materials needed to run the test.
- Efficient: Streamline workflow, testing for three pathogens with one simple procedure - saving your team critical time during the busy cold and flu season.
Product Documents
Healgen® COVID-19/Flu A&B Ag Combo Rapid Test Cassette
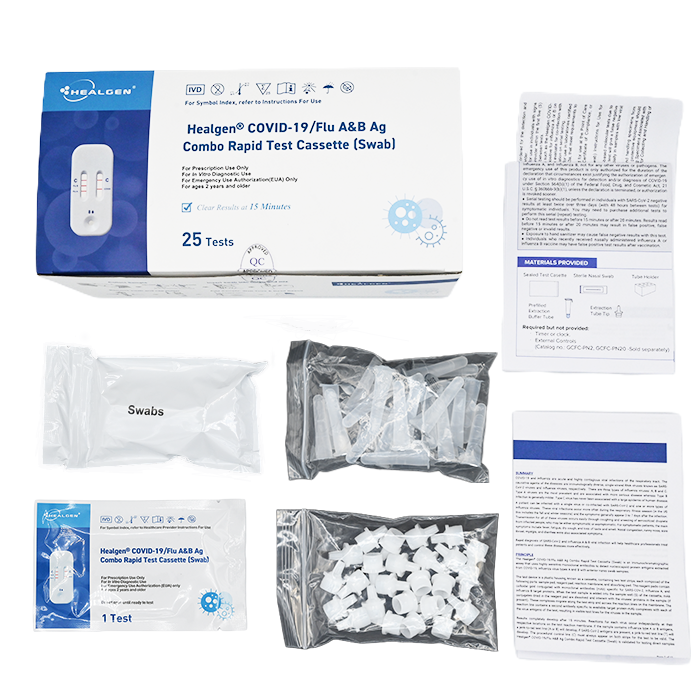
- 25 Sealed Test Cassette
- 25 Sterile Nasal Swabs
- 25 Prefilled Extraction Tube
- 25 Extraction Tube Tip
- 2 Tube Holders
- 1 Package Insert
- 1 Quick Reference Guide (QRG)
The Healgen® COVID-19/Flu A&B Ag Combo Rapid Test Cassette (swab) is a lateral flow immunochromatographic assay intended for in vitro rapid, simultaneous qualitative detection and differentiation of influenza A and influenza B nucleoprotein antigens and SARS-CoV-2 nucleocapsid antigen directly from anterior nasal swab specimens.
The Healgen® COVID-19/Flu A&B Ag Combo Rapid Test Cassette (swab) is intended for use in individuals who are suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider within the first five (5) days of symptom onset when tested at least twice over three days with at least 48 hours between tests.
Suitable for testing individuals 2+ years old. Swabbing should be performed by an adult for children aged 2 to 13. User must be aged 14 + to perform self test.
Authorized Laboratories: Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests. This product is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
| Product | Item Code | Quantity |
| COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab) | GCFC-525Sa | 25 Tests |
| External control: COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab) | GCFC-PN2 | 1 Each negative and positive control swab |
| External control: COVID-19/Flu A&B Ag Combo Rapid Test Cassette (Swab) | GCFC-PN10 | 10 Each negative and positive control swab |
Features
- 3-in-1 Test: Detects antigens from three common viral respiratory infections: COVID-19, Influenza A and Influenza B.
- Optimized Care: In just 15 minutes, test patients from a single swab sample and quickly determine the appropriate course for treatment during the same visit.
- Highly Sensitive:
92.0% sensitivity, 99.0% specificity for COVID-19.
92.5% sensitivity, 99.9%specificity for Flu A.
90.5% sensitivity, 99.9% specificity for Flu B. - Easy & Convenient: Uses non-invasive anterior nasal swab samples and the kit includes all materials needed to run the test.
- Efficient: Streamline workflow, testing for three pathogens with one simple procedure - saving your team critical time during the busy cold and flu season.
Product Documents
- The Healgen® COVID-19/Flu A&B Ag Combo Rapid test Cassette (Swab) has not been FDA cleared or approved but has been authorized by FDA under an EUA for use by authorized laboratories.
- This product has been authorized only for the detection of proteins from SARS-CoV-2, influenza A and influenza B, not for any other viruses or pathogens.
- The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.

 Canada
Canada