




Omicron Variant Update: Click Here to read our latest update on the impact of the Omicron variant of SARS-CoV-2 on COVID-19 Antigen Rapid Test Device.
The Rapid Response™ COVID-19 Antigen Rapid Test Device is an in vitro immunochromatographic assay for the direct and qualitative detection of SARS-CoV-2 viral nucleoprotein antigens from nasal and nasopharyngeal secretions from individuals suspected of COVID-19 within 6 days of symptom onset and from individuals without symptoms or other epidemiological reasons to suspect COVID-19 infection, when tested twice over two (or three) days with at least 24 hours (and no more than 36 hours) between tests. This test is authorized for use at the Point of Care i.e., in patient care setting. This product is available in both 5 tests per kit and 25 test per kit sizes.
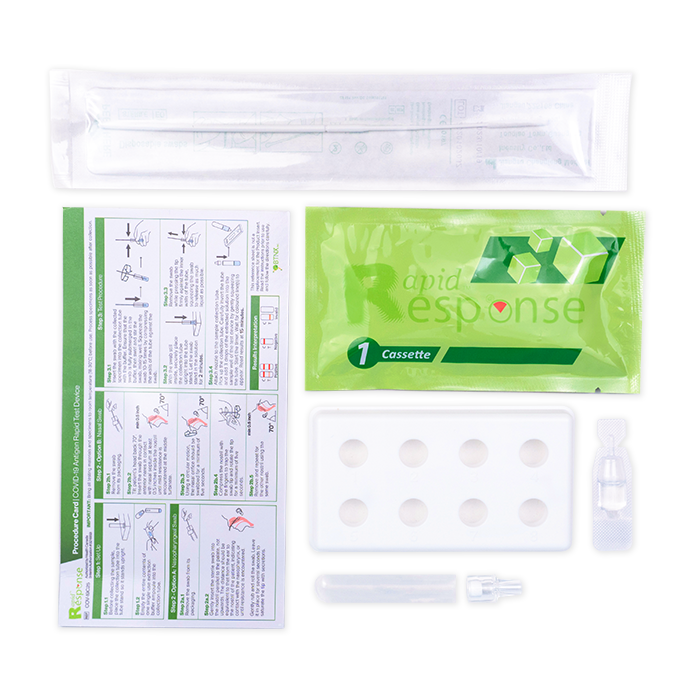
COVID-19 Antigen Rapid Test Device
- 5 Individually Packed Test Devices
- 5 Extraction Buffer
- 5 Extraction Tubes
- 5 Nozzles with Filters
- 1 Tube Stand
- 5 Individually Packed Swabs
- Product Insert
Omicron Variant Update: Click Here to read our latest update on the impact of the Omicron variant of SARS-CoV-2 on COVID-19 Antigen Rapid Test Device.
The Rapid Response™ COVID-19 Antigen Rapid Test Device is an in vitro immunochromatographic assay for the direct and qualitative detection of SARS-CoV-2 viral nucleoprotein antigens from nasal and nasopharyngeal secretions from individuals suspected of COVID-19 within 6 days of symptom onset and from individuals without symptoms or other epidemiological reasons to suspect COVID-19 infection, when tested twice over two (or three) days with at least 24 hours (and no more than 36 hours) between tests. This test is authorized for use at the Point of Care i.e., in patient care setting. This product is available in both 5 tests per kit and 25 test per kit sizes.
Pursuant to section 5 of the Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19, made by the Minister of Health on March 18, 2020, the Rapid Response™ COVID-19 Antigen Rapid Test Device is now authorized for sale or importation in Canada. Interim Authorization Number: 321669
For information on purchase orders please contact your local sales representative or contact us at covid19@btnx.com or 1-888-339-9964.
- Please note that certain products may only be available in specific regions; kindly consult with a sales representative for further information regarding product availability.
- The information provided on this website is for educational purposes only and should not be construed as medical advice. Always consult with a qualified healthcare professional regarding any medical concerns or conditions.
- Our products are intended for use as specified in the product documentation. It is important to carefully read and follow all instructions provided with the product.

 Canada
Canada