








Description
The Rapid Response® Strep A Rapid Test Device is a rapid test for the presumptive detection of Group A Streptococcus antigens in human throat swab specimens. This kit is intended for use as an aid in the diagnosis of Strep A infection directly from throat swabs to help diagnose and administer therapy immediately.
- Rapid test for detecting Strep A infection.
- Controls included in kit.
- Includes all materials needed to perform the test.
- Comes with a sturdy, plastic tube stand for mess-free workspace.
- Read results in 5 minutes.
- No specialized training or equipment required.
- Uses easy-to-collect throat swab specimens.
- No cross-reactivity with 53 common respiratory tract organisms.
- For point-of-care in vitro diagnostic use only.
Infectious Diseases
Strep A Rapid Test Device (Test Strips with Controls)
STR-15STC25
25 Tests/Kit
Need help? Contact our support or sales team.
Product Code:
STR-15STC25
Sample:
Throat Swab
Format:
Strip
Quantity:
25 Tests/Kit
Sensitivity:
97.4%
Specificity:
99.4%
Accuracy:
98.5%
Time to result:
5 minutes
Storage Condition:
2-30°C/36-86°F
Test Principle:
Lateral Flow Immunoassay
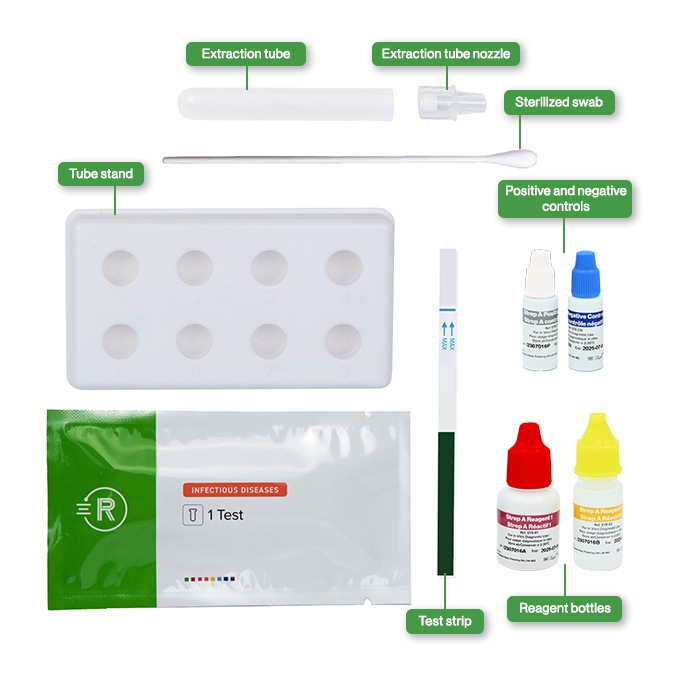
Contents
- 25 Individually packed test devices
- 1 Bottle of Reagent 1
- 1 Bottle of Reagent 2
- 25 Sterilized swabs
- 25 Nozzle with filter
- 25 Extraction tubes
- 1 Tube stand
- 1 Positive control
- 1 Negative control
- Package insert
Description
The Rapid Response® Strep A Rapid Test Device is a rapid test for the presumptive detection of Group A Streptococcus antigens in human throat swab specimens. This kit is intended for use as an aid in the diagnosis of Strep A infection directly from throat swabs to help diagnose and administer therapy immediately.
- Rapid test for detecting Strep A infection.
- Controls included in kit.
- Includes all materials needed to perform the test.
- Comes with a sturdy, plastic tube stand for mess-free workspace.
- Read results in 5 minutes.
- No specialized training or equipment required.
- Uses easy-to-collect throat swab specimens.
- No cross-reactivity with 53 common respiratory tract organisms.
- For point-of-care in vitro diagnostic use only.
Resources
References
- Facklam RR, Carey RB. Streptococci and Aerococci. In: Lennette EH, Balows A, Hausler WJ, Shadomy HJ, editors. Manual of Clinical Microbiology. 4th ed. Washington DC: American Society for Microbiology; 1985.
- Levinson ML, Frank PF. Differentiation of group A from other beta hemolytic streptococci with bacitracin. J Bacteriol. 1955 Mar;69(3):284-7.
- Edwards EA, Phillips IA, Suiter WC. Diagnosis of group A streptococcal infections directly from throat secretions. J Clin Microbiol. 1982 Mar; 15(3): 481-3.
- Gupta R, Talwar GP, Gupta SK. Rapid antibody capture assay for detection of group-A streptococci using monoclonal antibody and colloidal gold-monospecific polyvalent antibody conjugate. J Immunoassay. 1992; 13(3): 441-55.
- Ross PW. Throat swabs and swabbing technique. Practitioner. 1971 Dec; 207(242): 791-6.
- Lauer BA, Reller LB, Mirrett S. Effect of atmosphere and duration of incubation on primary isolation of group A streptococci from throat cultures. J Clin Microbiol. 1983 Feb; 17(2): 338-40.
Disclaimer
- Please note that certain products may only be available in specific regions; kindly consult with a sales representative for further information regarding product availability.
- The information provided on this website is for educational purposes only and should not be construed as medical advice. Always consult with a qualified healthcare professional regarding any medical concerns or conditions.
- Our products are intended for use as specified in the product documentation. It is important to carefully read and follow all instructions provided with the product.

 Canada
Canada