

Description
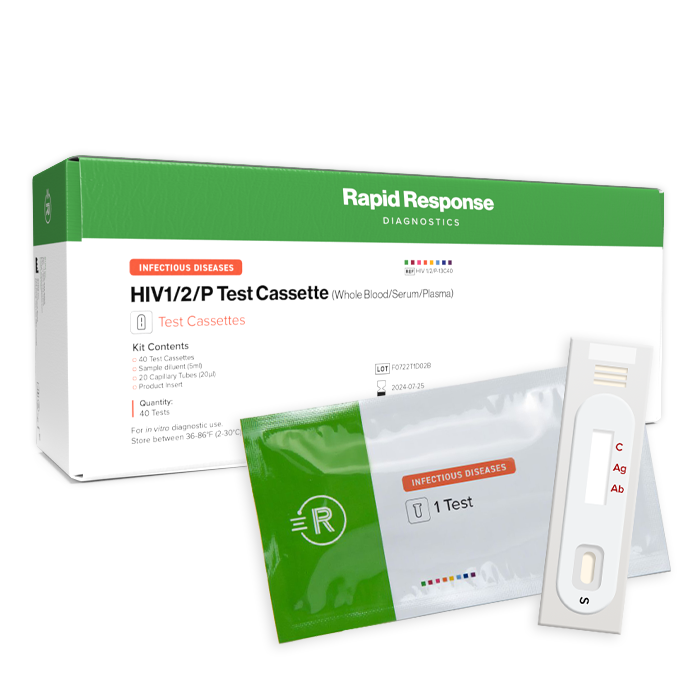
The Rapid Response™ HIV Ag/Ab 4th Generation Test Cassette is a lateral flow chromatographic immunoassay for the qualitative detection of antibodies (IgG, IgM, IgA) to anti-HIV- 1 (including O) and-2 virus and HIV-1 p24 antigen in human serum, plasma or whole blood. It is intended for professional in vitro diagnostic use only.
Interpretation of test results are: positive (two, or three lines), negative (one line), invalid (no lines).
Interpretation of test results are: positive (two, or three lines), negative (one line), invalid (no lines).
Infectious Diseases
HIV Ag/Ab (Antigen/Antibody) 4th Generation Test Cassette
HIV1/2/P-13C40L
40 Tests/Kit
Need help? Contact our support or sales team.
Product Code:
HIV1/2/P-13C40L
Sample:
Whole Blood / Serum / Plasma
Format:
Cassette
Quantity:
40 Tests/Kit
Time to result:
15 minutes
Storage Condition:
2-30°C/35.6-86°F
Test Principle:
Lateral Flow Immunoassay
Contents
- Individually packed test cassettes
- Sample diluent
- 20 μL capillary tubes
- Lancets (for whole blood)
- Product insert
Description
The Rapid Response™ HIV Ag/Ab 4th Generation Test Cassette is a lateral flow chromatographic immunoassay for the qualitative detection of antibodies (IgG, IgM, IgA) to anti-HIV- 1 (including O) and-2 virus and HIV-1 p24 antigen in human serum, plasma or whole blood. It is intended for professional in vitro diagnostic use only.
Interpretation of test results are: positive (two, or three lines), negative (one line), invalid (no lines).
Interpretation of test results are: positive (two, or three lines), negative (one line), invalid (no lines).
References
- . Chang, SY, Bowman, BH, Weiss, JB, et al. The origin of HIV-1 isolate HTLV-IIIB.Nature (1993) 3/363:466-9.
- Arya, SK, Beaver, B, Jagodzinski, L, et al. New human and simian HIV-related retroviruses possess functional transactivator (tat) gene. Nature (1987) 328:548-550.
- Caetano JA Immunologic aspects of HIV infection. Acta Med Port (1991) 4 Suppl1:52S-58S.
- Janssen, RS, Satten, GA, Stramer, SL, et, al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes.
JAMA(1998) 280(1): 42-4. - Busch, M.P., Lee, L.L., Satten, G.A., Henrard, D.R., Farzadegan, H., Nelson, K.E., Read, S., Dodd, R.Y and Peterson, L.R. Time course of detection of viral and serogic markers preceding human immunodeficiency virus type 1 seroconversion: implications for screening of blood and tissue donors. Transfusion (1995) 35:91-7.
Disclaimer
- Please note that certain products may only be available in specific regions; kindly consult with a sales representative for further information regarding product availability.
- The information provided on this website is for educational purposes only and should not be construed as medical advice. Always consult with a qualified healthcare professional regarding any medical concerns or conditions.
- Our products are intended for use as specified in the product documentation. It is important to carefully read and follow all instructions provided with the product.

 Canada
Canada