


Reference Number: RCHM-02071
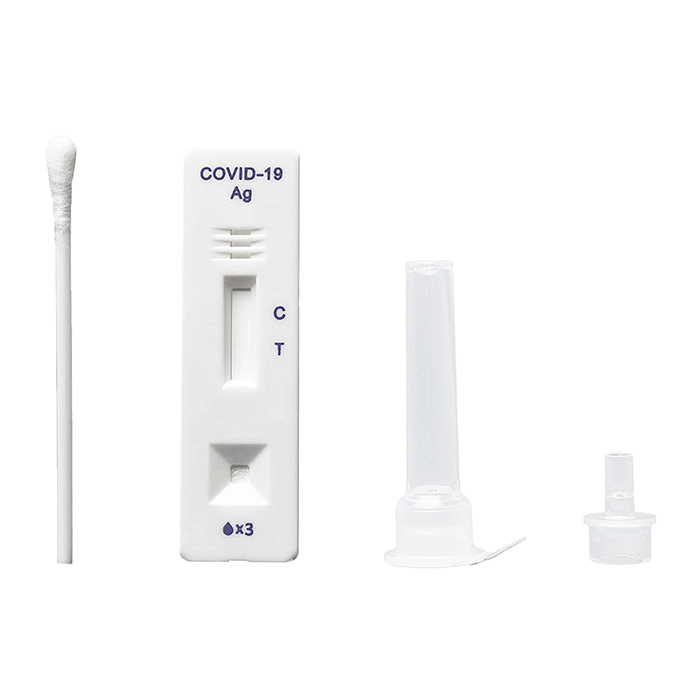
The CareStart™ COVID-19 Antigen test is a lateral flow immunochromatographic assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal or anterior nasal swab specimens directly collected from individuals who are either suspected of COVID-19 by their healthcare provider within first five days of symptom onset, or from individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests.
This test is authorized for use at the Point of Care (POC), operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
CareStart™ COVID-19 Antigen Test EUA CLIA Waived (USA)
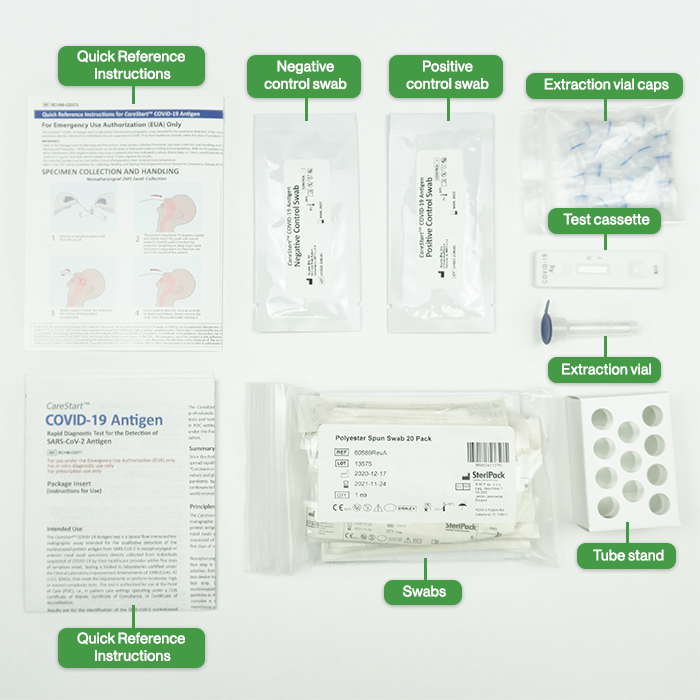
- Test Devices
- Extraction Vials/Caps
- Nasal (or nasopharyngeal) swab
- Positive Control Swab
- Negative Control Swab
- Package Insert
Reference Number: RCHM-02071
The CareStart™ COVID-19 Antigen test is a lateral flow immunochromatographic assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal or anterior nasal swab specimens directly collected from individuals who are either suspected of COVID-19 by their healthcare provider within first five days of symptom onset, or from individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests.
This test is authorized for use at the Point of Care (POC), operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
- This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA for use by authorized laboratories
- This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens
- The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
Please note: Certain CareStart COVID-19 Antigen tests currently labelled with expiration dates of July 2021 or earlier in fact have longer shelf lives than indicated by the expiration date on the label. A list of affected lots can be found at the following link. A copy of FDA’s letter to Access Bio regarding the shelf-life extension is available at the following link: https://www.fda.gov/media/150840/download.
- Please note that certain products may only be available in specific regions; kindly consult with a sales representative for further information regarding product availability.
- The information provided on this website is for educational purposes only and should not be construed as medical advice. Always consult with a qualified healthcare professional regarding any medical concerns or conditions.
- Our products are intended for use as specified in the product documentation. It is important to carefully read and follow all instructions provided with the product.
For information on the EUA, IFU, and purchase orders please contact your local sales representative or contact us at sales@lochnessmedical.com or 1-888-506-2658.

 Canada
Canada