COVID-19
An infectious disease is an illness caused by specific contact with a pathogenic viruses, bacteria or parasites. Diagnostic tests are vital tools in understanding and controlling its spread. BTNX Inc. offers an array of infectious diseases test kits that are geared to aid healthcare professionals and at home users in efficiently and accurately diagnosing many infectious diseases including COVID-19, RSV, FLU A & B, H. pylori and more!
Format: Pen
Kit Size: 20 Tests / Kit
Format: Pen
Kit Size: 10 Tests / Kit
Format: Pen
Kit Size: 5 Tests / Kit
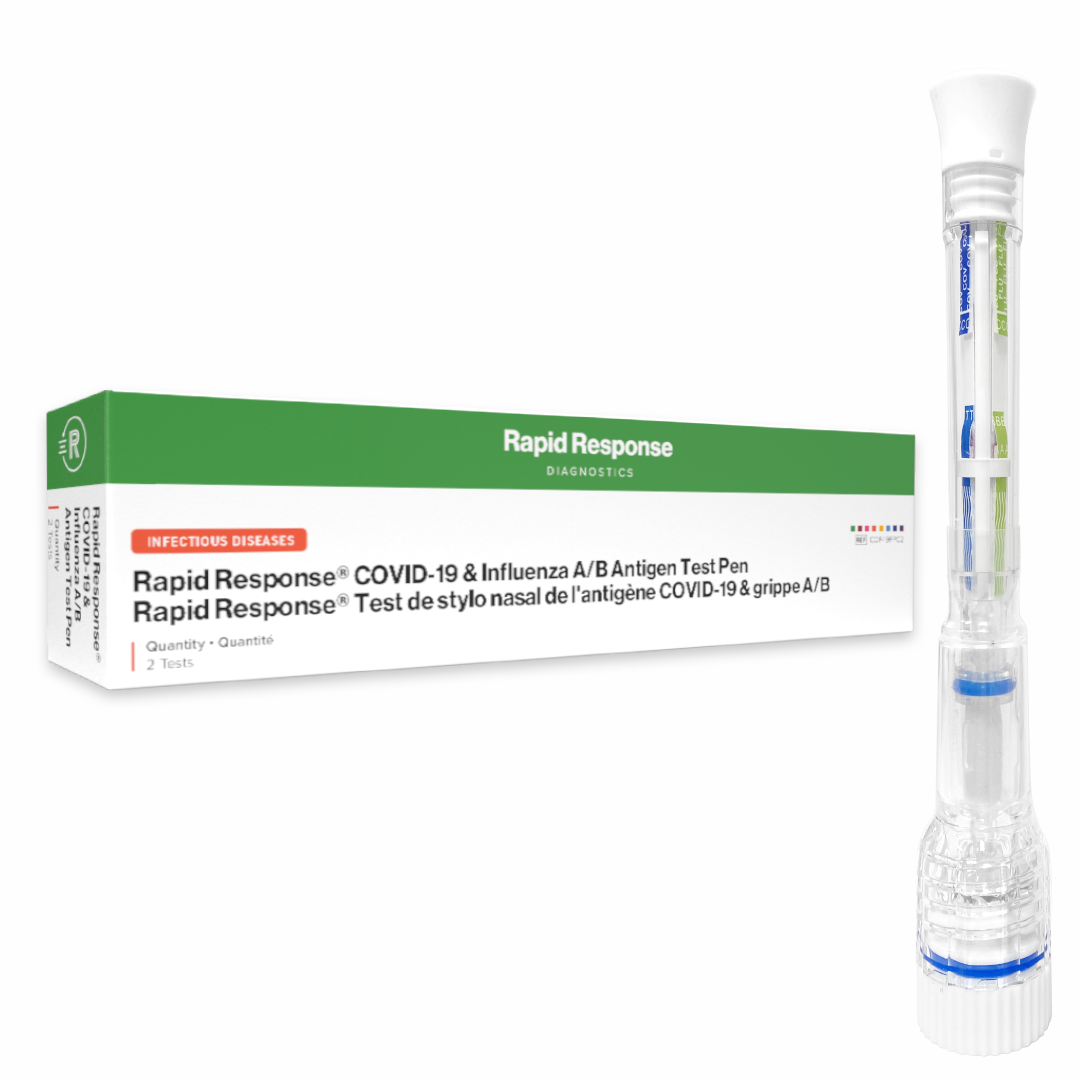
Format: Pen
Kit Size: 2 Tests / Kit
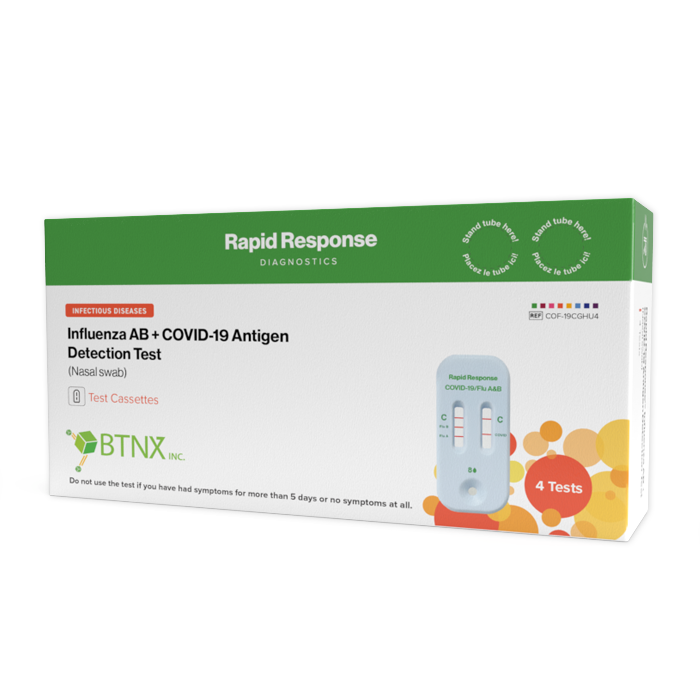
Format: Cassette
Kit Size: 4 Tests / Kit
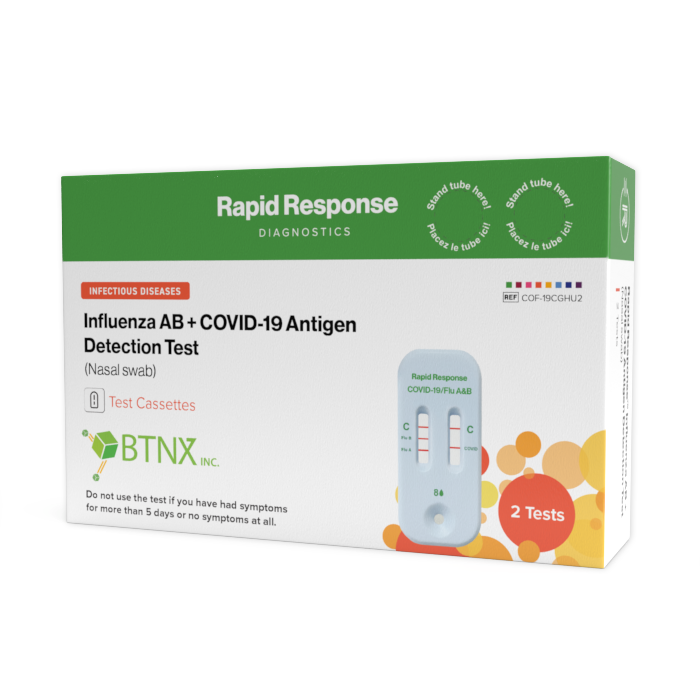
Format: Cassette
Kit Size: 2 Tests / Kit
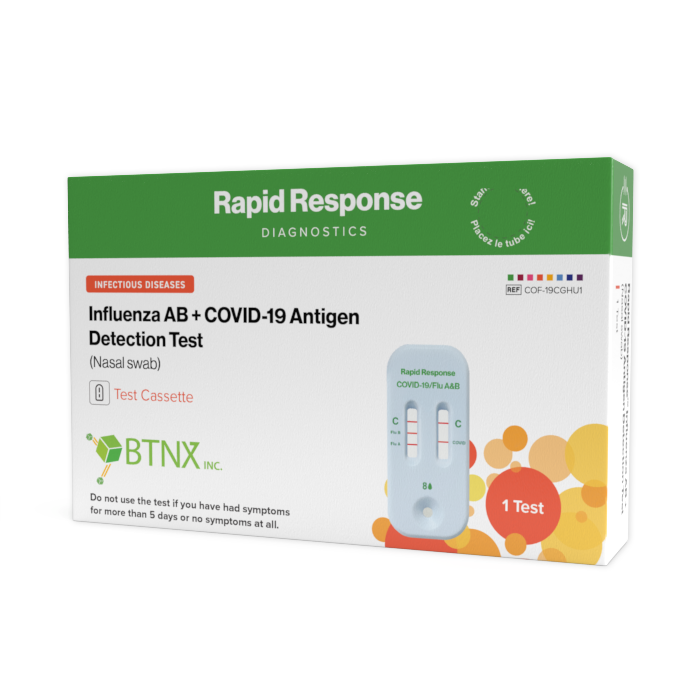
Format: Cassette
Kit Size: 1 Test / Kit
Format: Cassette
Kit Size: 25 Tests / Kit

Format: Cassette
Kit Size: 1 Test / Kit
Format: Cassette
Kit Size: 1 Test/ Kit

Format: Cassette
Kit Size: 2 Tests / Kit
Format: Cassette
Kit Size: 5 Tests / Kit
Format: Cassette
Kit Size: 10 Tests/ Kit
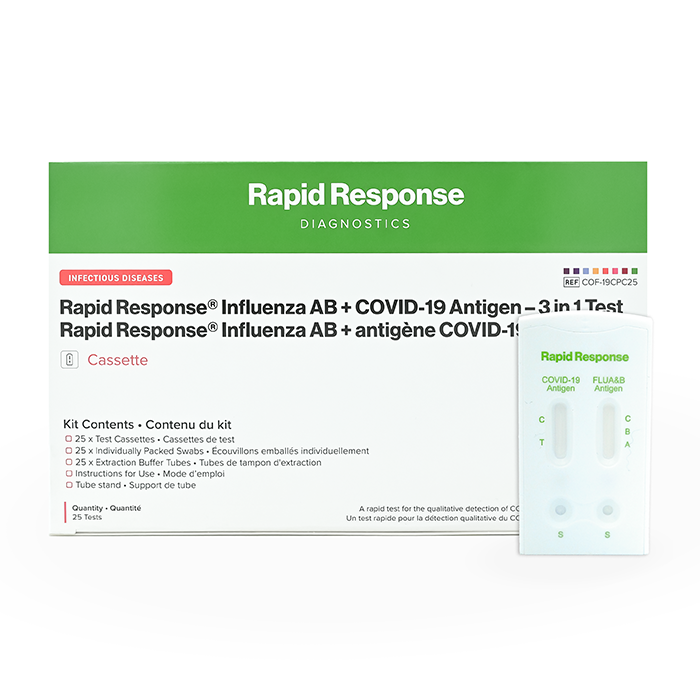
Format: Cassette
Kit Size: 25 Tests/Kit
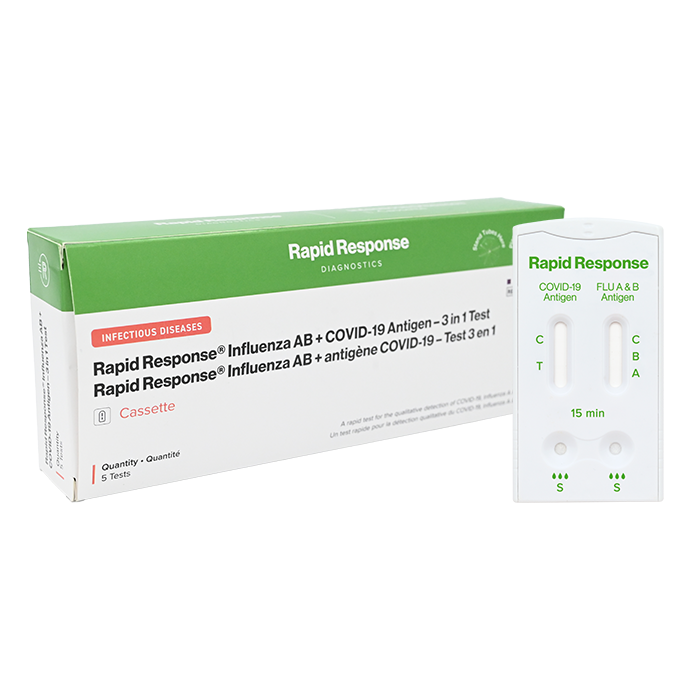
Format: Cassette
Kit Size: 5 Tests/Kit

Format: Cassette
Kit Size: 5 Test/Kit
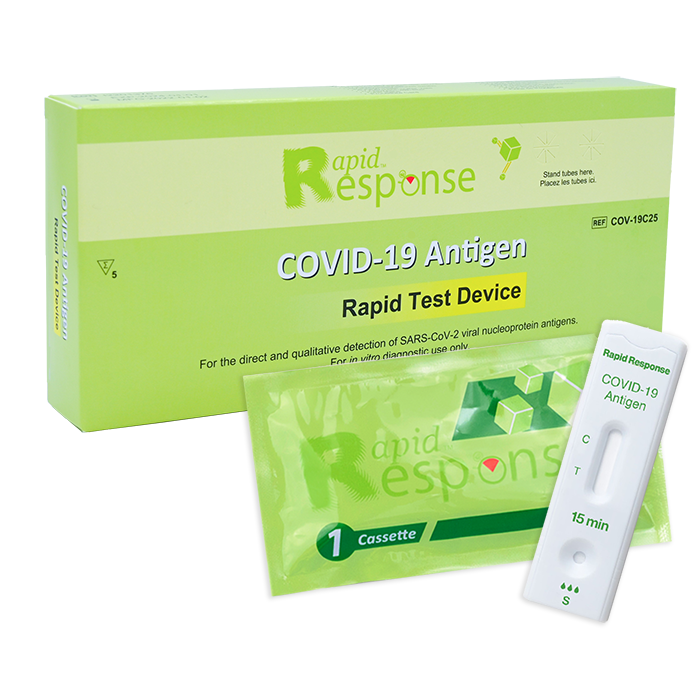
Format: Cassette
Kit Size: 25 Tests/Kit

Format: Cassette
Kit Size: 25 Tests/Kit

Format: Cassette
Kit Size: 5 Tests/Kit
.png)
Format: Cassette
Kit Size: 1 Test / Kit

Format: Cassette
Kit Size: 25 Tests / Kit
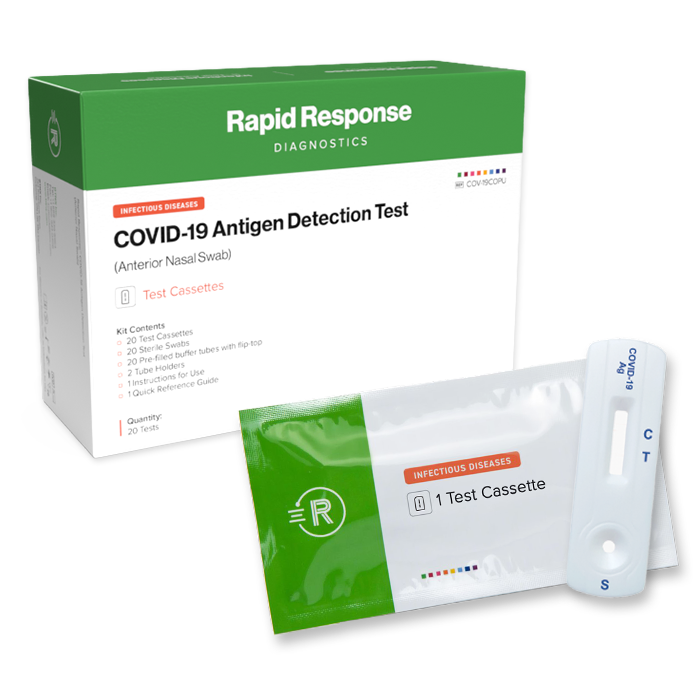
Format: Cassette
Kit Size: 20 Tests/Kit
Kit Size: 20 Tests/Kit

Format: Cassette
Kit Size: 10 Tests/Kit
Format: Cassette
Kit Size: 5 Tests/Kit
Format: Cassette
Kit Size: 25 Tests/Kit
Format: Cassette
Kit Size: 25 Tests/Kit
Overview of COVID-19 Testing Solutions
About COVID-19
COVID-19, caused by the novel SARS-CoV-2 virus and first identified in 2019, rapidly became a global pandemic, and was declared as such by the World Health Organization (WHO) on March 11, 2020. Infection by the disease is presented by a wide range of symptoms, varying from asymptomatic infection to severe respiratory illness, and in rare cases, death. It spreads primarily through respiratory particles, including those expelled by individuals who do not exhibit symptoms.
Belonging to the β-genus coronavirus, SARS-CoV-2 particles are round or elliptical in shape with a diameter of 60-140 nm. The virus is distinct from SARS-CoV and MERS-CoV and shares over 85% genetic similarity with bat SARS-like coronaviruses (bat-SL-CoVZC45).
SARS-CoV-2 primarily spreads through direct or close contact with infected individuals, as well as through respiratory droplets expelled during activities like coughing, sneezing, talking, or singing. Some evidence also suggests the possibility of indirect transmission through aerosol particles or contaminated surfaces. The incubation period typically ranges from 1 to 14 days, with the most common duration being 3 to 7 days.
The most common symptoms include fever, dry cough, and fatigue, but others like sore throat, headache, and muscle aches may also occur. High-risk groups include older adults and those with pre-existing medical conditions like cardiovascular disease, chronic respiratory issues, diabetes, or cancer, who are more likely to develop severe illness.
While not all infected individuals develop antibodies, tests detecting these antibodies can help gauge the pandemic’s reach and the population’s immune response. The duration of antibody persistence and their protective capacity remain uncertain.
The virus can spread not only when an individual is symptomatic but also before any noticeable symptoms appear. For a comprehensive list of COVID-19 symptoms, you can refer to the CDC's official website.
Understanding the nature of COVID-19 and its transmission is crucial in managing and combating this ongoing global health challenge.
What is a COVID-19 test kit?
A COVID-19 test kit is a rapid diagnostic tool designed to detect SARS-CoV-2 protein to confirm the presence of COVID-19.
Types of COVID-19 Tests
COVID-19 Diagnostic Tests
Diagnostic tests for COVID-19 are vital tools in understanding and controlling its spread. They can be broadly classified into:
- Molecular tests (like PCR tests) that detect the virus’ genetic material;
- Antigen tests that detect specific proteins from the virus; and
- Antibody tests that determine past infection by detecting antibodies.
Authorized At-Home OTC COVID-19 Tests
Rapid Response offers options for at-home tests that have received authorization for at-home over-the-counter (OTC) use. These are self-administered tests providing users with timely results without the need for laboratory processing.
OTC COVID-19 tests are designed for use by individuals at home without needing to send samples to a laboratory. They provide a convenient way to get quick results, aiding in timely decision-making.
Are home COVID-19 test kits accurate?
Our Rapid Response COVID-19 test kits are over 98% accurate.
How does a COVID-19 test kit work?
A COVID-19 test kit works by detecting the presence of the SARS-CoV-2 virus’ genetic material, nucleocapsid protein antigen, or anti-SARS-CoV-2 antibody in a sample, such as nasal/nasopharyngeal swab, blood, or saliva.
Antigen tests detect nucleocapsid protein antigen from SARS-CoV-2 virus from a sample often collected with a swab, which is then extracted in a buffer solution. The test strips contain antibodies that bind to COVID-19 antigens. When viral antigens are present, colored test lines appear. This type of test can help identify active infection.
Antibody tests detect anti-SARS-CoV-2 antibodies from a whole blood, serum, or plasma sample. The body produces antibodies in response to COVID-19 infection. This type of test can help identify past, and/or present, infection.
Where can I get a COVID-19 test kit?
Rapid Response COVID-19 test kits can be purchased on our website here. Our COVID-19 test kits are approved for home use, are easy to use with detailed instructions, and provide accurate results in just a few minutes.
Expiration Dates
Each COVID-19 rapid test kit comes with a specified expiration date, indicating its shelf life. Users must ensure the test is used before this date for accurate results. See individual product pages for details on shelf life.
Find the best solution for your needs
Our sales representatives can help set you up for success! Contact our team today!
Over the counter or home use COVID-19 test kits will include all necessary materials needed to perform the test, including a nasal/nasopharyngeal swab, extraction tube, workstation, and a test device. It is important to read the instructions provided with your test kit carefully before using the test, as not all COVID-19 test kits follow the same instructions.
Potential risks include possible discomfort during sample collection and errors in sample collection leading to possible incorrect results.
Potential benefits include the results, which along with other information, can help your healthcare provider make informed recommendations about your care. The results of this test may help limit the spread of COVID-19 to your family and others in your community.
If you have a positive test result, it is very likely that you have COVID-19 because proteins from the SARS-CoV-2 virus that causes COVID-19 were found in your sample. There is a very small chance that the test may produce a false positive result. Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. If you test positive, you should self-isolate and seek follow-up care with your healthcare provider. Additional testing may be necessary.
A negative test result indicates that COVID-19 virus was not detected. It is possible for the test to give a false negative result. An individual may still have COVID-19 even though the test is negative. The amount of antibodies or antigens may be below detectable levels in individuals who have been exhibiting symptoms for less than 15 days, or are not present during the stage of disease in which a sample is collected. A negative result does not preclude SARS-CoV-2 infection and should be confirmed via molecular assay (e.g., PCR). If you test negative but continue to have COVID-19 symptoms, seek follow up care from your healthcare provider.
Yes, our team follows research on new variants of COVID-19 to ensure that any changes in the variant will not impact the performance of the test. Both the Rapid Response COVID-19 Antigen Rapid Test Cassette – At Home (COV-19C25B) and Rapid Response COVID-19 Antigen Rapid Test Device (COV-19C25 / COV-19C5) were tested with Omicron variants BA.1, BA.2 BA.4 and BA.5 recombinant proteins and with positive samples, confirmed by sequencing. It was confirmed that both tests accurately detect these strains at or near the same limit of detection. Read a statement here [BA1-BA2-BA4-BA5-Statement-COV-19C25B-and-COV-19C25.pdf (btnx.com) ]
Individuals confirmed to have COVID-19 often exhibit symptoms of fever and/or symptoms of acute respiratory illness (e.g., cough, dyspnea), although some individuals experience only mild symptoms or no symptoms at all. Currently known symptoms include cough, shortness of breath or dyspnea, fever, chills, myalgias, headache, sore throat or new loss of taste or smell, nausea or vomiting or diarrhea. While the severity can range from mild to intense, some individuals infected with COVID-19 may have no symptoms at all. Signs and symptoms may appear any time from 2 to 14 days after exposure to the virus, and the average time to symptom onset is approximately 5 days.
Serial testing involves testing an individual for COVID-19 more than once. Since antigen tests are not as sensitive as other testing methods and can sometimes give false results, repeated testing is recommended to better identify cases. By repeating testing, it can help lead to faster detection of COVID-19 cases and reduce the spread of infection. Depending on the individual’s risk factors and test results, further molecular testing for COVID-19 may be required. It is important that you work with your healthcare provider to help you understand the next steps you should take.
Molecular tests, also referred to as PCR tests, identify the virus’ genetic material. In contrast, antigen tests detect proteins from the virus. Antigen tests are precise in detecting the virus but are not as sensitive as molecular tests. This means that a positive result is highly accurate, but a negative result does not necessarily rule out infection when using an antigen test.
Rapid COVID-19 test kits typically provide results in 10-15 minutes. Please read the instructions carefully with your kit for accurate results.
Time to Results:
- COV-19C1AD - 10 minutes
- COV-19C25AD - 10 minutes
Yes, the COVID-19 test is authorized for individuals with symptoms of COVID-19 within the first 7 days of symptom onset when tested at least twice over three days with at least 48 hours between tests, and for individuals without symptoms or other epidemiological reasons to suspect COVID-19, when tested at least three times over five days with at least 48 hours between tests.
COVID-19 test kits can be used for travel or before returning to work/school. It is important to test before traveling or social gathering to prevent spreading the virus.
Certain countries accept rapid antigen screening for inbound travel. Make sure your test is accepted in the country you are travelling to so you can avoid any complications.
COVID-19 test kits can be used as proof to return to work/school. Check with your work/school if your test is accepted.
Home test kits can be used to test family members or close contacts. Please read the product insert included with your kit for the authorized ages that can use the test and details on how to use your test kit for the specified ages.
Nasal swab tests involve inserting a swab into the nose to collect a sample either from the back (nasopharyngeal) or anterior portions of the nasal passage. They are commonly used because the virus tends to be present in higher quantities in the nasal passage, especially in the early stages of infection.
Saliva tests require a sample of saliva (oral fluid), which can be less invasive and more comfortable to collect than a nasal swab. However, the efficiency and accuracy of the test may vary. It is important to follow and read the instructions carefully provided with your kit for accurate results.
There are COVID-19 antigen test kits available for self-testing and on-site testing. PCR tests require a healthcare professional to administer and the collected test sample must be sent to a lab for analysis.
COVID-19 test are single use only and should not be reused.
Store COVID-19 test kits at 2-30°C (36-86°F) when not in use. The test device must remain in the sealed pouch until use. Do not use after the expiration date.
COVID-19 test kits should not be used on anyone under 2 years old. Children aged 2 and up should be tested by an adult. Children 14 and above may test themselves under adult supervision.
For questions or to report a problem, please call technical support at 1-888-339-9964 (MON-FRI 9AM – 5:30PM EST) or email support@btnx.com.

 Canada
Canada